Notes, Imp Points, Formulae ,etc.
of 12th chemistry chapter 2 solutions based on HSC Syllabus (NEW)
Introduction :
Recall -
Mixture : It is a combination of two or more substances.
Mixtures are :-
(a) Homogeneous : Mixing of components is uniform.
(b) Heterogeneous : Non-Uniform mixing of components.
Classification of Homogeneous Mixtures :-
1) Colloids 2) True Solutions
DEFINITION OF
Solution: The solution is a homogeneous mixture of two or more pure substances.
TYPES OF SOLUTIONS
There are total of 9 Types of Solutions :-
Capacity of Solution to Dissolve Solute
Definition of
Saturated Solution : It is the solution which contains maximum amount of solute dissolved in a given amount of solvent at a given temperature.
(Equilibrium can be formed)
Super Saturated Solution : A Solution containing greater than equilibrium amount of solute is said to be super saturated solution.
(Unstable Solution)
Solubility :
Definition : The solubility of a solute is its amount per unit volume of a saturated solution at a specific temperature.
FACTORS AFFECTING SOLUBILTY
Solubility depends on the nature of solute and solvent, temperature and pressure.
1) Nature of Solute and Solvent :-
LIKE DISSOLVES LIKE
- Polar solutes dissolve in polar solvents. (NaCl in Water)
- Non polar organic compounds dissolves in nonpolar solvents . (Cholesterol in benzene)
The substances having similar intermolecular forces are likely to be soluble in each other.
2) Effect of temperature on solubility :
- In endothermic process, when temperature is increased , the solubility of substance increases. (KCl in Water )
- In Exothermic process, the solubility decreases with increase in temperature. (CaCl2 in water)
- Solubility of gases decrease with increase in temperature
NOTE : There is no direct correlation between solubility and exothermicity and endothermicity.
2) Effect of pressure on solubility :
- Pressure has no effect on the solubilities of solids and liquids as they are incompressible.
- The solubility of gases increases with increasing pressure.
THE QUANTITATIVE RELATIONSHIP IS GIVEN BY HENRY'S LAW
Henry's Law :-
It states that the solubility of a gas in a liquid is directly proportional to the pressure of gas over the solution.
S - Solubility of the gas in mol/L
P - Pressure of the gas in bar over the solution.
Kh - The proportionality constant is called Henry's Law Constant.
Exceptions to Henry's Law
Gases like NH3 and CO2 do not obey Henry's Law.
Reason : Because these gases react with water.
Reason : Because these gases react with water.
2.5 Vapour pressure of solutions of liquids in liquids
Raoult’s law : It states that the partial vapour pressure of any volatile component of
a solution is equal to the vapour pressure of the pure component multiplied by its mole fraction in the solution.
Suppose that for a binary solution of two volatile liquids A1 and A2 , partial pressures p1 and p2 and their mole fractions x1 and x2 respectively.
Composition of Vapour Phase:
To find composition of vapour,
Let y1 and y2 be mole fractions of two components in vapour, p1 and p2 are their partial pressures and P is total vapour pressure.
then,
p1 = y1p p2 = y2p
2.5.2 Ideal and nonideal solutions
1. Ideal solutions
i. Ideal solutions obey Raoult’s law over entire range of concentrations.
ii. No heat is evolved or absorbed when two components forming an ideal solution are
mixed. Thus, the enthalpy of mixing is zero. ∆mix H = 0
iii. There is no volume change when two components forming an ideal solution are
mixed. Thus volume of an ideal solution is equal to the sum of volumes of two
components taken for mixing. ∆mix V = 0
iv. In an ideal solution solvent-solute, solute- solute and solvent-solvent interactions are comparable.
v. The vapour pressure of ideal solution always lies between vapour pressures of
pure components.
2. Nonideal solutions
i. These solutions do not obey Raoult’s law over the entire range of concentrations.
ii. The vapour pressures of these solutions can be higher or lower than those of pure
components.
iii. Deviation from the Raoult's law : These solutions show two types of deviation
from the Raoult's law.
A. Positive deviations from Raoult's Law
Positive Deviation : Vapour pressure of solution greater than those of pure components.
Condition : Solute-solvent intermolecular attractions weaker than solute-solute and solvent-solvent intermolecular attractions.
Example :
The solutions of ethanol and acetone, carbon disulphide and acetone
B. Negative Deviations from Raoult's Law
Negative Deviation : Vapour pressure of solution smaller than those of pure components.
Condition : Solute-solvent intermolecular attractions stroger than solute-solute and solvent-solvent intermolecular attractions.
Example :
The solutions of phenol and aniline, chloroform and acetone
2.6 Colligative properties of nonelectrolyte solutions :
Colligative Property : The physical properties of solutions that depend on the number of solute particles in solutions and not on their nature are called
colligative properties.




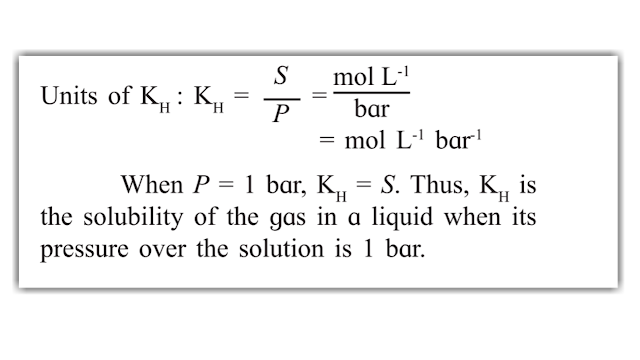





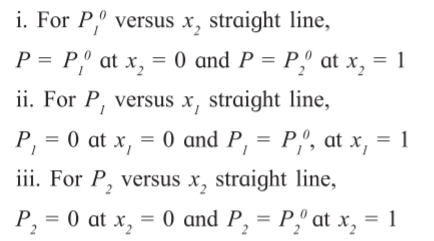












Plese upload the remained part of the solution chapter
ReplyDeleteEk number nites
DeleteThanks for these notes
ReplyDeletethank you for giving us these notes
ReplyDeleteSir plz upload notes of these chapter only from 2.8.2 with exercise plzzz sir I need ur help plzz help us
Thank u sir
Nice Blog. Thanks for sharing with us. Such amazing information.
ReplyDeleteTop Boarding School in Uttarakhand
Thnx for these notes I hope this will surely help me out👍😊
ReplyDeleteThanku very much sir
ReplyDeleteGreat and useful
ReplyDeleteVisit https://chemistry--solutions.blogspot.com
ReplyDeleteLanguage of the notes are very easy for understanding.... Thank you 😊
ReplyDeleteThank you for notes
ReplyDeleteThanx
ReplyDelete